Folded or cut, this lithium-sulfur battery keeps powering devices
by Clarence Oxford
Los Angeles CA (SPX) Sep 19, 2024
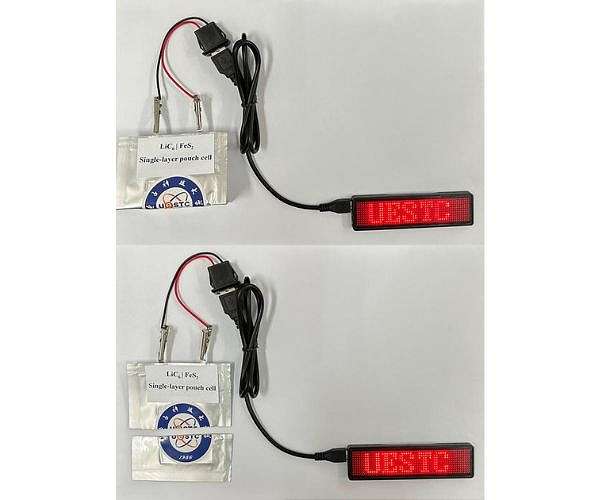
Most rechargeable batteries that power portable devices, such as toys, handheld vacuums and e-bikes, use lithium-ion technology. But these batteries can have short lifetimes and may catch fire when damaged. To address stability and safety issues, researchers reporting in ‘ACS Energy Letters’ have designed a lithium-sulfur (Li-S) battery that features an improved iron sulfide cathode. One prototype remains highly stable over 300 charge-discharge cycles, and another provides power even after being folded or cut.
Sulfur has been suggested as a material for lithium-ion batteries because of its low cost and potential to hold more energy than lithium-metal oxides and other materials used in traditional ion-based versions. To make Li-S batteries stable at high temperatures, researchers have previously proposed using a carbonate-based electrolyte to separate the two electrodes (an iron sulfide cathode and a lithium metal-containing anode). However, as the sulfide in the cathode dissolves into the electrolyte, it forms an impenetrable precipitate, causing the cell to quickly lose capacity. Liping Wang and colleagues wondered if they could add a layer between the cathode and electrolyte to reduce this corrosion without reducing functionality and rechargeability.
The team coated iron sulfide cathodes in different polymers and found in initial electrochemical performance tests that polyacrylic acid (PAA) performed best, retaining the electrode’s discharge capacity after 300 charge-discharge cycles. Next, the researchers incorporated a PAA-coated iron sulfide cathode into a prototype battery design, which also included a carbonate-based electrolyte, a lithium metal foil as an ion source, and a graphite-based anode. They produced and then tested both pouch cell and coin cell battery prototypes.
After more than 100 charge-discharge cycles, Wang and colleagues observed no substantial capacity decay in the pouch cell. Additional experiments showed that the pouch cell still worked after being folded and cut in half. The coin cell retained 72% of its capacity after 300 charge-discharge cycles. They next applied the polymer coating to cathodes made from other metals, creating lithium-molybdenum and lithium-vanadium batteries. These cells also had stable capacity over 300 charge-discharge cycles. Overall, the results indicate that coated cathodes could produce not only safer Li-S batteries with long lifespans, but also efficient batteries with other metal sulfides, according to Wang’s team.
Research Report:Chelating-Type Binders toward Stable Cycling and High-Safety Transition-Metal Sulfide-Based Lithium Batteries
Related Links
American Chemical Society
Powering The World in the 21st Century at Energy-Daily.com